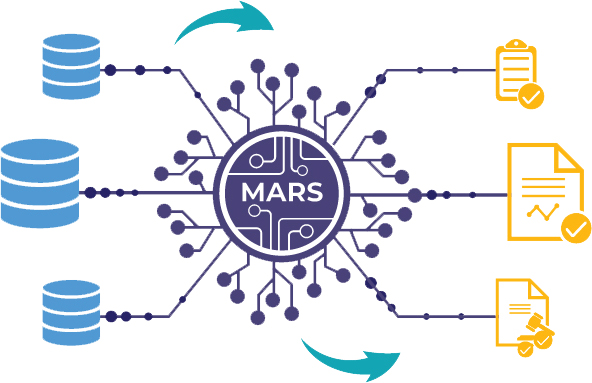
MARS integrates content and regulatory intelligence applying organizational specific business processes to generate optimal commitments schedule and regulatory deliverable components eliminating guesswork. It explores, analyzes and constructs compliance and quality variables into workflows designed for efficient resource utilization, homing in on triggers that improve overall quality and regulatory compliance.
- Solution exclusively designed for Life Sciences Industry
- 21 CFR Part 11 Compliance
- Cloud & On-Premise Support
- 60% External Cost Savings
- 50% FTE effort Optimization
- Reduction in error rates
- Compliance and Audit Readiness
Automated Content Management
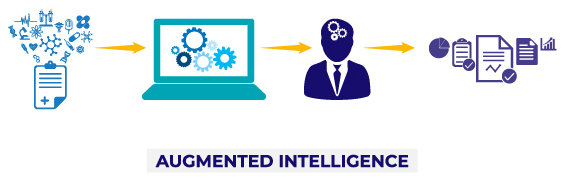
Quartica’s ACM is a unique approach that blends the most cutting-edge elements of automation and digitization technologies and combines them with the power of the human brain, an approach generally known as augmented intelligence. Quartica’s Automated Content Management solutions keep life sciences users in the driver’s seat while empowering them with tools to accelerate business processes and driving overall quality and compliance indicators. With the application of Automated Content Management the end user is still fundamental to the final output but is now able to deliver at a speed, scope and quality that could only be achieved with the aid of intelligent machine computing.
MARS ADVANTAGES

Automated authoring of documents and reports
Automated authoring to generate documents and reports. Content generated from various data sources applying rules, regulations and templates

No re-tooling and or significant training required
End users continue to operate in the familiar MS-Word authoring environment. Ability to leverage existing processes and tools as needed requiring minimal change management

Automated generation from existing content
Intelligent linking of content for re-use in future versions and derivative documents enabling consistency and traceability.

Inspection and audit ready metrics and reports
Full audit trail and logs for usage in inspections and audits. Tracking of revisions for intelligent future content optimization
MARS DIFFERENTIATORS

Business Process Management

Electronic Document Management

Quality and Regulatory Compliance

Business Intelligence & Reporting
MARS CONFIGURATIONS

Data Sources

Regulatory Intelligence

Templates

Workflows
MARS DEPOLYMENT

Cloud Based

Enterprise on Premise
Discover your clear path to automation excellence using MARS
REQUEST DEMOIndustry Standard Validated Solution
Key Differentiators
- Solution developed exclusively for life sciences domain unlike cross industry platforms
- Automation of business processes combining content using regulatory intelligence
- Automation of regulatory compliance and quality metrics and reports
- Business and information architecture encapsulating key domain concepts in the clinical domain allowing sharing, re-use and extension of information concepts
- Solution modules developed iteratively in close collaboration business end users
- Modules successfully implemented and in production use representing cost savings, compliance improvement and ROI
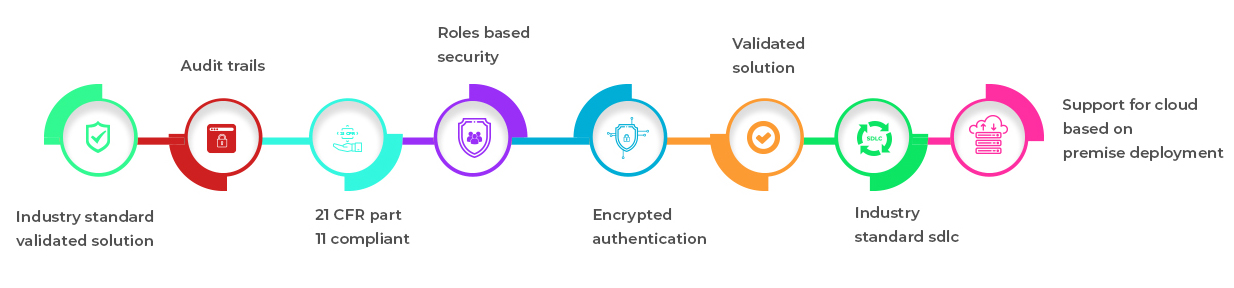
Industry Standard Validated Solution
- 21 CFR Part 11 Compliant
- Audit Trails
- Roles based security
- Encrypted authentication
- Validated Solution
- Industry Standard SDLC
- Support for cloud based or on premise deployment

